|
Prandin
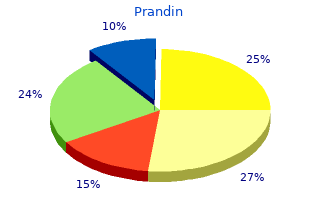
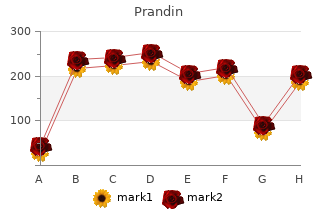
2018, Aurora University, Jared's review: "Prandin generic (Repaglinide) 2 mg, 1 mg, 0.5 mg. Proven Prandin.".
Peripheral vein nutrition is a less optimal form of feeding in that adequate caloric support cannot be achieved except in unusual circumstances generic prandin 1 mg online diabetic alert dogs. Consequently prandin 1 mg on line diabetes signs wiki, it is seldom used except where there are no other options or during the transition phase to full enteral feeding status. Complications of Parenteral Feeding: Tolerance to parenteral feedings should be evaluated throughout the course. In that acute parenteral nutrition is most common in patients who are critically ill, considera- tion always must be given to fluid status as well as glucose intolerance and electrolyte abnormalities. An acute shift toward anabolism may unmask preexisting body electrolyte deficiencies (see Monitoring Progress and Complications, below. Abnormalities of acid–base balance also occur more frequently in such patients, and alterations in electrolyte compo- sition (such as acetate salts) of solutions may be indicated. As always, patients with indwelling catheters must be monitored carefully for 58 S. An abrupt change in glucose tolerance may indicate infection related to the catheter or another source. Problems Related to Access These problems can be life-threatening and include misadventures related to placement of enteral or parenteral feeding portals. Acute pneumothorax, inadvertent arterial puncture, air embolism, and per- foration of the vena cava or heart can accompany attempts at central venous access. Insertion of catheters by experienced personnel serves to minimize these complications. More frequently, however, it is the initial misplacement of the catheter or latent events such as insertion-site infection or vessel thrombosis that provide troubling morbidities to patients. These complications are monitored by a rigorous adherence to sterility guidelines and protocols and by regular physical examination of the patient. A constant awareness of the potential for these events promotes early intervention and treatment. Problems related to placement of enteral feeding portals arise with similar, if not greater, frequency. Although it is increasingly popular to return to intragastric feeding, proper tube placement and function also must be assured. Problems of aspiration, especially in patients prone to reflux, may preclude this route of enteral nutrient provision. Under such circumstances, the placement of small-bore feeding catheters either transgastrically or transcutaneously requires experienced per- sonnel. As noted above, enteral feeding tubes may cause abdominal distention or symptoms that must be investigated. Careful, daily physical examination is an essential component of the monitoring regimen. Problems related to access portals as well as organ dys- function and fluid imbalance may be detected initially, or solely, on this basis. A determination of red blood cell indices may help to define iron deficiency (not routinely provided in intravenous nutrition). Eval- uation of basic bleeding parameters is undertaken to detect the pres- ence of vitamin K deficiency, which also may develop in parenterally fed patients. Trace mineral deficiencies may be a latent problem, especially in patients with preexisting malnutrition and prolonged inflammatory conditions. Attention should be given to patients with previous compromise of intestinal absorption. Problems of Excess Significant changes in overall clinical status as well as specific organs may provoke a state of excess provision. At least daily evalua- tion of glucose tolerance, by blood or urine sampling, is indicated in all patients. An abrupt increase in glucose levels in an otherwise stable patient must suggest infection until proven otherwise. Glucose excess also may precipitate or aggravate pulmonary prob- lems in some patients. If the rate of endogenous glucose oxidation is exceeded, carbon dioxide retention may result in respiratory distress or weaning problems in ventilated patients.
Pharmacokinetic study of piperacillin in newborns relating to gestational and postnatal age order prandin 0.5mg mastercard diabetes symptoms 7 dpo. Human renal function maturation: a quantitative description using weight and postmenstrual age purchase prandin 0.5mg mastercard diabetes gad test. In vitro activity and pharmacodynamics of commonly used antibiotics against adult systemic isolates of Escherichia coli and Pseudomonas aeruginosa at 40 U. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. Metronidazole population pharmacokinetics in preterm neonates using dried blood-spot sampling. Simultaneous determination of 5 beta-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high- performance liquid chromatography with ultraviolet detection. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. Clinical data by gestational age group Gestational age at birth (weeks) Characteristic <26 26–29 30–32 N 29 20 7 Gestational age, weeks 24 (22, 25) 27 (26, 29) 31 (28, 32) Postnatal age, days 17 (1, 77) 15 (1, 75) 24 (3, 65) Postmenstrual age, weeks 27 (23, 35) 30 (27, 38) 35 (31, 40) Weight, g 709 (400, 1400) 1023 (555, 2580) 1610 (1357, 1890) Female sex 14 (48) 5 (25) 6 (86) Race White 15 (52) 10 (50) 4 (57) Black 12 (41) 9 (45) 2 (29) Other 2 (7) 1 (5) 1 (14) Hispanic 2 (7) 1 (5) 2 (29) Serum creatinine (mg/dL) 1 (0. Visual predictive check of piperacillin dose-normalized concentrations versus time. Solid and dashed black lines represent observed and predicted median concentrations, respectively. Weight-normalized piperacillin clearance versus serum creatinine (A) and body weight (B). One proposed method is the use of scavenged samples left over from the normal clinical care of infants. Food and Drug Administration for the treatment of adults with serious infections caused by susceptible anaerobic bacteria but is not approved for use in children. In spite of this, metronidazole is extensively used “off-label” in 2 children to treat anaerobic intra-abdominal infections (i. In young infants, its use is typically restricted to treatment of rare cases of anaerobic bacteremia, central nervous system infections, and complicated intra-abdominal infections 3,4 such as necrotizing enterocolitis. Because infection in young infants with very low birth weight (<1500 g birth weight) is associated with devastating outcomes including death and neurodevelopmental impairment, appropriate dosing recommendations for agents such as metronidazole are needed in this population. These recommendations are derived from small, single-center studies and have not been prospectively evaluated. In addition, these dosing regimens are cumbersome due to the different combinations of maturation components required to choose the most appropriate dose. Metronidazole dosing was determined by the routine clinical practice in each unit, and no exclusion criteria were used. The study was approved by the institutional review boards at each institution, and informed consent was obtained from a parent or guardian prior to enrollment. Missing weights were imputed with the last recorded value carried forward for up to 7 days. Scavenged samples were defined as samples obtained 66 without obtaining additional blood from the infant. Blood draw samples were defined as samples obtained with collection of extra blood from the infant. The duration of metronidazole infusion was performed according to site routine clinical care. Samples were refrigerated or placed on ice immediately after collection and then centrifuged at 1500 g and o 4 C for 10 minutes. Samples from all sites were shipped on dry ice to Duke University Medical Center where they were stored at -70° C prior to analysis. The first-order conditional estimation method with interaction was used for all model runs. Once covariates were identified during the model- 68 building process, covariate testing was performed via standard forward addition backward elimination methods. A forward inclusion with backwards elimination approach was used during the multivariable step, and a reduction of 6. Model evaluation Models were evaluated based on successful minimization, goodness-of-fit plots, precision of parameter estimates, bootstrap procedures, and visual predictive check. For the visual predictive check, the final model was used to generate 1000 Monte Carlo simulation replicates of metronidazole exposure, and simulated results were compared with those observed in the study. The number of observed concentrations outside the 90% prediction interval for each time point was quantified.
|