|
Isoptin
2018, Concord College, Arokkh's review: "Isoptin 240 mg, 120 mg, 40 mg. Purchase cheap Isoptin no RX.".
Infections: Corticosteroids may mask some signs of infection buy cheap isoptin 40mg line pulse pressure nursing, and new infections may appear during their use buy cheap isoptin 40 mg on line heart attack kit. Blood pressure: Average and large doses of hydrocortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids. Drugs that induce hepatic enzymes such as phenobarbital, phenytoin and rifampin may increase the clearance of corticosteroids and may require increases in corticosteroid dose to achieve the desired response. Dermatologic: Impaired wound healing; thin fragile skin; petechiae and ecchymoses; facial erythema; increased sweating; may suppress reactions to skin tests. Neurological: Convulsions; increased intracranial pressure with papilloedema (pseudotumour cerebri) usually after treatment; vertigo; headache. Endocrine: Menstrual irregularities; development of Cushingoid state; suppression of growth in children; secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness; decreased carbohydrate tolerance; manifestations of latent diabetes mellitus; increased requirements for insulin or oral hypoglycaemic agents in diabetics. As a quaternary ammonium derivative, hyoscine-N- butylbromide does not enter the central nervous system. Therefore, anticholinergic side effects at the central nervous system do not occur. Peripheral anticholinergic effects result from a ganglion-blocking action within the visceral wall as well as from anti- muscarinic activity. Concomitant treatment with dopamine antagonists such as metoclopramide may result in diminution of the effects of both drugs on the gastrointestinal tract. Reduction of respiratory tract and oral secretions (particularly in the palliative setting). Note, for this indication, hyoscine butylbromide is preferred by the palliative care team. The syndrome of nasal polyps, angioedema, and bronchospastic reactivity to aspirin or other nonsteroidal anti-inflammatory agents. Bleeding Risk: Ibuprofen, like other nonsteroidal anti-inflammatory agents, can inhibit platelet aggregation but the effect is quantitatively less and of shorter duration than that seen with aspirin. Ibuprofen has been shown to prolong bleeding time (but within the normal range) in normal subjects. Because this prolonged bleeding effect may be exaggerated in patients with underlying haemostatic defects, ibuprofen should be used with caution in persons with intrinsic coagulation defects and those on anticoagulant therapy. Renal Effects: As with other nonsteroidal anti-inflammatory drugs, long-term administration of ibuprofen to animals has resulted in renal papillary necrosis and other abnormal renal pathology. In humans, there have been reports of acute interstitial nephritis with haematuria, proteinuria, and occasionally nephrotic syndrome. A second form of renal toxicity has been seen in patients with prerenal conditions leading to a reduction in renal blood flow or blood volume, where the renal prostaglandins have a supportive role in the maintenance of renal perfusion. In these patients administration of a nonsteroidal anti-inflammatory drug may cause a dose dependent reduction in prostaglandin formation and may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and the elderly. These abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. Severe hepatic reactions, including jaundice and cases of fatal hepatitis, have been reported with ibuprofen as with other nonsteroidal anti-inflammatory drugs. Although such reactions are rare, if abnormal liver tests persist or worsen, if clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e. Aseptic Meningitis Aseptic meningitis with fever and coma has been observed on rare occasions in patients on ibuprofen therapy. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have an underlying chronic disease. During concomitant therapy with ibuprofen, the patient should be observed closely for signs of renal failure, as well as to assure diuretic efficacy. Lithium: Ibuprofen produces an elevation of plasma lithium levels and a reduction in renal lithium clearance in patients on concomitant therapy.
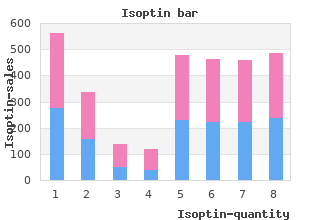
Philadelphia: Chemical Heritage Press discount isoptin 40 mg otc arrhythmia when sleeping, 1999; Wolfgang Wimmer buy isoptin 240mg without a prescription pulse pressure less than 10, “Wir haben fast immer was Neues”: Gesundheitswesen und Innovationen der Pharma-Industrie in Deutschland. Vietor, How Countries Compete: Strategy, Structure, and Government in the Global Economy. Despite an ongoing international harmonization process for pharmaceuticals, the past decade has seen the continuation and even expansion of these national regulatory systems. The comparison of the United States and Germany specifcally, and the United States and Europe more generally that is developed in this chapter illuminates tensions associated with consumer-driven regulation that are shaping the competitive landscape for pharmaceutical product development and sales. It is striking that just as patient and disease-based activists have taken on certain regulatory functions traditionally associated with the state or peak medical associations, a greater consumer and market orientation in medical care has increasingly put the onus on patients to independently seek out information about pharmaceuticals and to treat prescription drugs like other goods they purchase. However, little scholarship to date has connected these developments with the industry’s economic performance or business strategy. Though in part speculative at this point, the data and analysis presented here are an initial step toward deepening the understanding of interrelationships among government regulation, patients’ mobilization both as regulators and consumers, and the functioning of the pharmaceutical industry. First, I present summary data and brief analysis of the pharmaceutical sector in its national contexts. Some critics of the industry have argued that by chasing global markets and by moving research and manufacturing facilities to countries with lower labor costs, frms were able to shop for weaker national regulatory systems and exert deregulatory pressure. Instead, it appears that the pharmaceutical industry has remained largely concentrated in the United States and Europe, which historically set high barriers to drug approvals. Second, the chapter seeks to connect academic literature in the history and economics of innovation with studies of regulation. Both areas have seen interesting work in recent years, but little has been done to integrate perspectives from the two areas. Dukes, “The Regulation of Drugs: Worlds of Difference” International Journal of Technology Assessment in Health Care. The data suggests a puzzle of why frms headquartered in the United States have had notably better results since the early 1980s than their competitors in Germany, and to a lesser extent other European countries. A regulatory approach defned around protecting “patients” in Europe, and the complexities of shared authority among the medical profession, other peak associations, and the state in Germany may have unwittingly contributed to a weaker domestic pharmaceutical industry. The conclusion argues that understanding the relationship of innovation to regulation in different countries is critical to moving beyond current crises in regulatory policy to the beneft of patients for whom medicines are intended. Locating Pharmaceutical Production and Consumption In the late 1970s and early 1980s, a series of studies by the U. Yet some thirty years later, a set of similar measures presented here suggests that the U. New York: United Nations, 1979; National Academy of Engineering, The Competitive Status of the U. Thomas, “Estimating the Effects of Regulation on Innovation: An International Comparative Analysis of the Pharmaceutical Industry,” Journal of Law and Economics 21 (1978), 133-163; for a review of the drug lag, see: Arthur Daemmrich, “Invisible Monuments and the Costs of Pharmaceutical Regulation: Twenty-Five Years of Drug Lag Debate,” Pharmacy in History 45 (2003), 3-17. Additional measures, including the size of the national pharmaceutical market and the attractiveness of a country for clinical research, help to deepen this analysis and connect to the discussion of regulatory approaches in the face of a new disease, societal pressures for compassionate use programs, and attention to biomarkers as a component of a personalized approach to medicine that follows in the next section. In a striking development considering the industry’s origins in Germany, France, and Switzerland, in the past ffteen years have witnessed a signifcant shift in the center of power of the pharmaceutical industry: of the ffteen largest global frms in 005, nine were headquartered in the United States, whereas one was in France, two in Switzerland, and the sole German frm to make the group came in the fourteenth position. Through the mid-1980s, the balance was rather more evenly distributed: even though only three of the top ffteen frms were based in Germany, two of them – Hoechst and Bayer – held the top two positions. All of the leading frms expanded international markets in this three-decade period, however, sales fgures correlate well with new product innovation and frms headquartered in the United States moved from the bottom half toward the top of the list between 1974 and the present. Table 1: Top 15 Pharmaceutical Firms by Sales, 19749 Rank Company Name Location Pharmaceutical Sales ($ millions) 1 Roche Switzerland 1,386 2 Merck U. In addition to this shift in position for frms based on their headquarters location, another striking feature of these tables is the phenomenal growth in sales for top frms between 1988 and 2005, compared to more modest growth during the 1970s and early 1980s. For many of the top frms, this growth was achieved through mergers and heavy marketing of new products. Yet at least half of the top ffteen companies did not achieve growth through mergers and instead expanded sales signifcantly based on new product introductions alone. More generally, the nearly ten-fold sales growth between 1988 and 2005 indicates the degree to which pharmaceuticals have become high-demand consumer products.
Were records of the products used available as dosage form discount isoptin 240mg fast delivery arrhythmia medication list, strength isoptin 120mg sale blood pressure levels in adults, batch number, expiry date, certificate of analysis, other coding that identifies the specific characteristic of the product tested? Was the shipping letter of the test and reference products from the sponsor to the investigator available? Were the records of delivery and receipt of the test and comparator products available? Were the records kept under conditions that will prevent deterioration including protection from fire? Documentation of file movements Inspection preliminary conclusion based on the Total of findings number of findings Critical finding (s) Major finding (s) Other finding (s) Conclusion Are additional information requested as a follow up of the inspection to reach a conclusion? Area additional information requested as a follow up of the assessment to reach a conclusion? The programme was subsequently expanded to include evaluations of carcinogenic risks associated with exposures to complex mixtures, life-style factors and biological and physical agents, as well as those in specific occupations. The objective of the programme is to elaborate and publish in the form of monographs critical reviews of data on carcinogenicity for agents to which humans are known to be exposed and on specific exposure situations; to evaluate these data in terms of human risk with the help of international working groups of experts in carcinogenesis and related fields; and to indicate where additional research efforts are needed. Application for rights of reproduction or translation, in part or in toto, should be made to the International Agency for Research on Cancer. Inclusion of an agent in the Monographs does not imply that it is a carcinogen, only that the published data have been examined. Equally, the fact that an agent has not yet been evaluated in a monograph does not mean that it is not carcinogenic. The evaluations of carcinogenic risk are made by international working groups of independent scientists and are qualitative in nature. Anyone who is aware of published data that may alter the evaluation of the carcino- genic risk of an agent to humans is encouraged to make this information available to the Unit of Carcinogen Identification and Evaluation, International Agency for Research on Cancer, 150 cours Albert Thomas, 69372 Lyon Cedex 08, France, in order that the agent may be considered for re-evaluation by a future Working Group. Although every effort is made to prepare the monographs as accurately as possible, mistakes may occur. Readers are requested to communicate any errors to the Unit of Carcinogen Identification and Evaluation, so that corrections can be reported in future volumes. Ferguson, Auckland Cancer Society Research Centre, Faculty of Medical and Health Science, The University of Auckland, Private Bag 92019, Auckland 1000, New Zealand S. Turusov, Blokhin Cancer Research Centre, Russian Academy of Medical Sciences, Kashirskoye Shosse 24, 115478 Moscow, Russian Federation V. Wilbourn, Unit of Carcinogen Identification and Evaluation (Responsible Officer) Technical assistance M. The Monographs programme has since been expanded to include consideration of exposures to complex mixtures of chemicals (which occur, for example, in some occupations and as a result of human habits) and of exposures to other agents, such as radiation and viruses. The Monographs represent the first step in carcinogenic risk assessment, which involves examination of all relevant information in order to assess the strength of the avai- lable evidence that certain exposures could alter the incidence of cancer in humans. Detailed, quantitative evaluations of epidemio- logical data may be made in the Monographs, but without extrapolation beyond the range of the data available. Quantitative extrapolation from experimental data to the human situation is not undertaken. The term ‘carcinogen’ is used in these monographs to denote an exposure that is capable of increasing the incidence of malignant neoplasms; the induction of benign neo- plasms may in some circumstances (see p. The aim of the Monographs has been, from their inception, to evaluate evidence of carci- nogenicity at any stage in the carcinogenesis process, independently of the underlying mechanisms. The Monographs may assist national and international authorities in making risk assessments and in formulating decisions concerning any necessary preventive measures. These evaluations represent only one part of the body of information on which regulatory measures may be based. Other components of regulatory decisions vary from one situation to another and from country to country, responding to different socioeconomic and national priorities. Therefore, no recommendation is given with regard to regulation or legislation, which are the responsibility of individual governments and/or other international organizations.
|